A) polymerization
B) combustion
C) oxidation
D) reduction
E) double displacement
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many structural isomers possessing the alcohol functional group have the molecular formula C3H8O?
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
A peptide bond is the amide linkage that is formed in a condensation reaction involving the __________ group of one amino acid with the carboxylic acid group of a second amino acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A saturated hydrocarbon is
A) a hydrocarbon that contains oxygen.
B) a compound in which all carbon atoms have four single bonds.
C) a compound in which one or more carbon atoms have double or triple bonds.
D) a hydrocarbon that is dissolved in water.
E) a cycloalkane with five or more carbons.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of hydrocarbon is the following molecule? 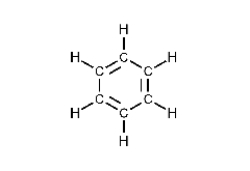
A) alkane
B) alkene
C) alkyne
D) aromatic
E) cycloalkene
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions is a substitution reaction?
A) C6H6 + Cl2 → C6H5Cl + HCl
B) CH2=CH2 + Cl2 → CH2ClCH2Cl
C) CH2=CH2 + HCl → CH3CH2Cl
D) CH2=CH2 + H2 → CH3CH3
E) HC
CH + 2Cl2 → CHCl2CHCl2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an aldehyde is oxidized, the product is a(n) ____.
A) ester
B) ketone
C) ether
D) alcohol
E) carboxylic acid
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The addition of Br2 is used as the reaction to distinguish between alkanes and alkenes. What is the observation that accompanies this test?
A) A bright red color is produced when Br2 reacts with an alkene.
B) Bromine dissolves in alkanes but not in alkenes.
C) Bromine is a dark red liquid. When it adds to the double bond of an alkene to make the dibromide, it becomes colorless.
D) The red color of bromine disappears when it dissolves in alkanes.
E) Bromine reacts with alkanes to form a precipitate.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the general formula of an alkane?
A) CnH2n+2
B) C2nH2n-2
C) CnHn
D) CnH2n
E) Cn+2Hn
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds might be used to reduce a carboxylic acid to an aldehyde?
A) H+
B) Cl2
C) K2Cr2O7
D) KMnO4
E) NaBH4
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture of ethanol and benzoic acid is heated in the presence of an acid. Which of the following is the primary product of this reaction?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the products of a condensation reaction between a primary amine and a carboxylic acid?
A) water and an amide
B) water and an ester
C) ammonia and an amide
D) ammonia and an ester
E) an ammonium cation and a carboxylate anion
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw Lewis structures for all possible isomers of C3H6O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alcohols is least likely to be soluble in water?
A) 3-pentanol
B) 2-butanol
C) 1,2-ethanediol
D) 1,2,3-propanetriol
E) Methanol
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the two types of stereoisomers?
A) geometrical and optical
B) optical and positional
C) geometrical and positional
D) geometrical and functional group
E) chiral and optical
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the product of the addition of excess F2 to acetylene?
A) 1,2-difluoroethane
B) Ethylene
C) 1,1,2,2-tetrafluoroethane
D) Ethane
E) 1,1-difluoroethylene
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formulas represents an unsaturated hydrocarbon?
A) C2H6
B) C3H8
C) C4H10
D) C6H12
E) C7H16
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the following benzene derivative? 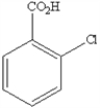
A) 1-chlorobenzoic acid
B) 5-chloroanaline
C) 1-acetate-1-chlorobenzene
D) 5-chlorobenzoic acid
E) 1,3-chlorocarboxylic benzene
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct systematic name for the following alkene? 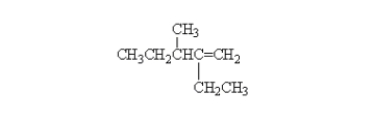
A) 2-ethyl-3-methyl-2-hexene
B) 2-ethyl-3-methyl-1-pentene
C) 4-ethyl-3-methyl-4-pentene
D) 3-methyl-4-ethyl-4-pentene
E) octene
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
In vulcanized rubber, the polymer chains of natural rubber are cross-linked with sulfur atoms. The polymer chains stretch when stressed, but they return to their original shape when the stress is removed. Substances that behave this way are called ________.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 88
Related Exams