Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures is different from the other three? 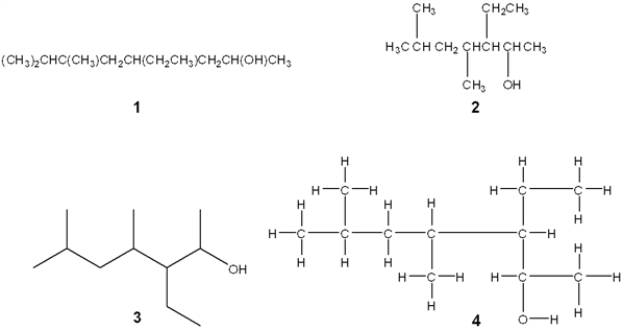
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 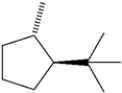
A) trans-1-isopropyl-4-methylcyclopentane
B) cis-1-tert-butyl-2-methylcyclopentane
C) trans-1-tert-butyl-2-methylcyclopentane
D) cis-1-isopropyl-2-methylcyclopentane
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the IUPAC name of the following compound? 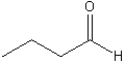
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 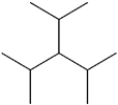
A) 2,4-dimethyl-3-isopropyl-pentane
B) 3-isopropyl-1,5-dimethylpentane
C) 3-isopropyl-2,4-dimethylpentane
D) triisopropylmethane
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups?
A) 1,1-dimethylcyclohexane
B) cis-1,2-dimethylcyclohexane
C) trans-1,2-dimethylcyclohexane
D) cis-1,3-dimethylcyclohexane
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 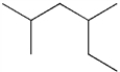
A) 2-ethyl-4-methylpentane
B) 2,4-dimethylhexane
C) 3,5-dimethylhexane
D) 1,1,3-trimethylpentane
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is "X" in the following reaction? ? 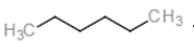
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 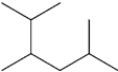
A) 2-isopropyl-5-methylpentane
B) 5-isopropyl-2-methylpentane
C) 2,3,5-trimethylhexane
D) 1,2-diisopropylpropane
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is "X" in the following reaction? ?
A)
O
||
B)
C)
O
||
D)
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Approximately how long is a C−C single bond of an alkane?
A) 111 pm
B) 134 pm
C) 142 pm
D) 153 pm
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram. 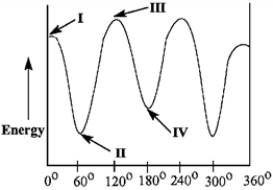
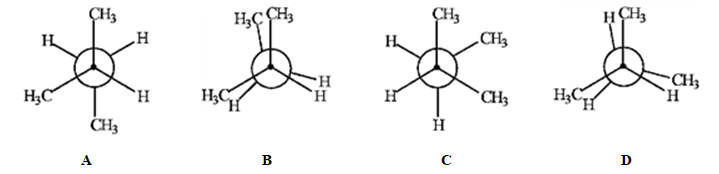 -Conformation A is represented by Roman numeral ____.
-Conformation A is represented by Roman numeral ____.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 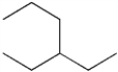
A) 3-propylpentane
B) 1,1-diethylpropane
C) 3-ethylhexane
D) isooctane
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the constitutional isomer with the molecular formula C7H16. 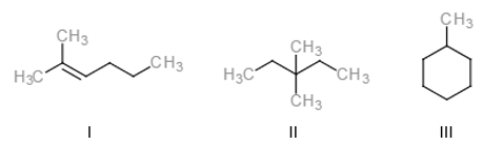
A) Only I
B) Only I and II
C) Only II
D) I, II, and III
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures represents trans-1,3-dimethylcyclohexane? 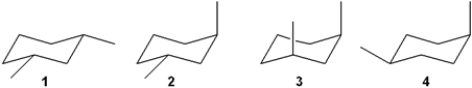
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram. 
 -Conformation C is represented by Roman numeral ____.
-Conformation C is represented by Roman numeral ____.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of molecular oxygen (O2) are consumed in the complete combustion of one mole of hexane (C6H14) ?
A) 6
B) 9.5
C) 12.5
D) 14
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a Newman projection of the most stable conformation of 3-methylpentane, CH3CH2CH(CH3)CH2CH3 looking along the C2-C3 bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many constitutional isomers are there with the molecular formula C6H14?
A) 3
B) 4
C) 5
D) 8
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? ![What is the IUPAC name for the following compound? A) cycloheptane B) bicyclo[3.2.0]heptane C) bicyclo[5.4]heptane D) cyclobutylcyclopentane](https://d2lvgg3v3hfg70.cloudfront.net/TB7078/11ead7c8_9dd3_1a29_84b0_8dc4b52b810f_TB7078_00.jpg)
A) cycloheptane
B) bicyclo[3.2.0]heptane
C) bicyclo[5.4]heptane
D) cyclobutylcyclopentane
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 101
Related Exams