Correct Answer

verified
Correct Answer
verified
True/False
The percent s character in an sp2 hybridized orbital is approximately 33%.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
The formal charge on carbon in carbon monoxide is______.
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the alkanes that have the formula C5H12.
Correct Answer

verified
Correct Answer
verified
Essay
Circle and name the functional groups in the following molecule. 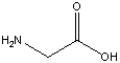
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an ionic bond?
A) Br−Br
B) C−Cl
C) C−S
D) Na−O
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are there in the valence shell of the nitrogen atom of ammonia?
A) 4
B) 5
C) 6
D) 8
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ammonia (NH3) and which contain the lone pair of electrons. Label each orbital with its hybridization.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding resonance structures?
A) Each resonance structure is in rapid equilibrium with all of the other structures
B) The resonance structures may have different energies
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must have the same number of electrons
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds has the smallest dipole moment?
A) Li−Cl
B) C−H
C) O−H
D) H−Cl
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a sodium cation (sodium: atomic number 11) ?
A) 1s22s22p63s1
B) 1s22s22p53s1
C) 1s22s22p6
D) 1s22s22p63s2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the H−C−H bond angles in a methyl anion, CH3−?
A) 90°
B) 109°
C) 120°
D) 180°
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the H−C−H bond angles in a methyl cation, CH3+?
A) 90°
B) 109°
C) 120°
D) 180°
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The approximate H-C-H bond angle in methane is ______°.
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the tertiary (3°) amines that have the formula C5H11N.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has an atom that has an unfilled valence shell of electrons?
A) molecular hydrogen, H2
B) hydroxide anion, HO−
C) boron trifluoride, BF3
D) water, H2O
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The following molecule contains an _____________functional group. 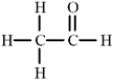
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons can the shell with a principal quantum number of 1 hold?
A) 1
B) 2
C) 4
D) 8
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following shows curved arrows that correctly accounts for the differences between the two structures? 
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a carboxylic acid?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) CH3CH2CHO
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 118
Related Exams