A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2COOCH3
D) CH3CH2COCH3
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the given ion given, what is the formal charge of nitrogen, carbon, and sulfur, respectively? 
A) -2, +1, and 0.
B) 0, -2, and +1.
C) -2, 0, and +1.
D) +1, 0, and -2.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-carbon σ molecular bonding orbital of ethyne, HC≡CH?
A) C2p + C2p
B) C2sp + C2sp
C) C2sp2 + C2sp2
D) C2sp3 + C2sp3
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents the shape of a 2p atomic orbital of carbon? 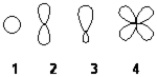
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a ketone?
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate H−C−O bond angle in formaldehyde, H2C=O?
A) 90°
B) 109°
C) 120°
D) 180°
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a tertiary (3°) alcohol?
A) 1
B) 2
C) 3
D) 4
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a primary amine?
A) CH3CH2NHCH3
B) CH3CH2NHCH(CH3) 2
C) CH3CH2N(CH3) 2
D) (CH3) 3CNH2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
Overlap of the two atomic orbitals as shown could result in the formation of a π bond. 

B) False
Correct Answer

verified
Correct Answer
verified
Essay
Circle all of the sp2 hybridized atoms in the following molecular structure. 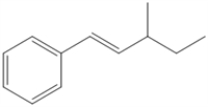
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 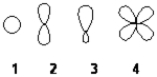
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
What is the molecular formula of aspartame, shown below? 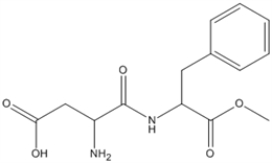
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding resonance structures?
A) All resonance structures must have the same number of electrons
B) Each atom in all of the resonance structures must have a complete shell of valence electrons
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must be valid Lewis structures
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the circled bonds is the strongest? 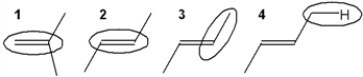
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The formal charges in the complex should below are 0 on each H, -1 on N, and +1 on B. 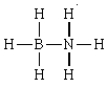
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following resonance structures is the least important contributor to the resonance hybrid of the acetate anion, CH3COO−? 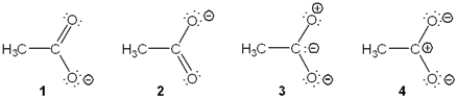
A) 1
B) 2
C) 3
D) 4
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents the shape of the 2s atomic orbital of carbon? 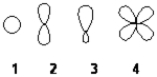
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a nitrogen atom (nitrogen: atomic number 7) ?
A) 1s22s12p4
B) 1s22s22p3
C) 1s12s12p5
D) 1s22s22p2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 118 of 118
Related Exams