A) α decay.
B) β decay.
C) γ decay.
D) electron capture.
E) spontaneous fission.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following nuclei has a magic number of neutrons and/or protons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
B
Correct Answer
verified
True/False
Gamma rays are not deflected by an electric field.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon-14 will emit a β particle with an energy of 0.1565 MeV. What is this energy in joules?
A) 1.0 × 10 -24 J
B) 2.5 × 10 -20 J
C) 1.0 × 10 -18 J
D) 2.5 × 10 -14 J
E) None of these choices are correct.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioisotope 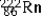 will decay through
will decay through
A) α decay.
B) �β decay.
C) γ decay.
D) positron decay.
E) electron capture.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Gamma rays are high energy electrons.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 30.0-kg child receives 2.65 × 107 β particles, each with an energy of 4.60 × 10-13 J. If the RBE = 0.78, how many millirem did the child receive?
A) 3.2 × 10 -7
B) 5.2 × 10 -7
C) 5.2 × 10 -4
D) 3.2 × 10 -2
E) None of these choices are correct.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The (negative) binding energy per nucleon reaches a maximum for the isotope 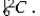
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotopes 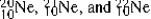 are all stable, while
are all stable, while 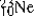 is radioactive. The mode of decay for
is radioactive. The mode of decay for 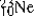 is most likely to be
is most likely to be
A) positron decay.
B) α decay.
C) γ decay.
D) electron capture.
E) β decay.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gamma-rays and X-rays interact with matter, causing
A) formation of ions, but no free radicals.
B) formation of free radicals, but no ions.
C) nuclear transmutation reactions.
D) formation of ions and free radicals.
E) formation of ions and nuclear transmutation reactions.
G) A) and E)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Calcium-39 undergoes positron decay. Each positron carries 5.49 MeV of energy. How much energy will be emitted when 0.0025 mol of calcium-39 decays?
A) 13.2 kJ
B) 1.32 × 10 4 kJ
C) 1.32 × 10 6 kJ
D) 1.32 × 10 9 kJ
E) None of these choices are correct.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exposure to 10 nCi for 10 minutes is more hazardous for a child than for an adult because
A) the child's cells are dividing more rapidly than the adult's and are, therefore, more susceptible to the radiation.
B) the child's smaller body size makes the effective dose larger for the child than for the adult.
C) the child's immune system is not developed well enough to resist damage.
D) the child's skin is not as thick as an adult's and cannot block as much radiation.
E) None of these choices are correct.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The difference between the rad and the rem is
A) the rem is a rad per year.
B) the rad takes into account the type of radiation.
C) the rem takes into account the effect on the particular biological tissue.
D) the rem is a rad per kilogram.
E) None of these choices are correct.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 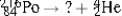
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
An alkaline earth element is radioactive. It and its daughter elements decay by emitting a total of three alpha particles in succession. In what group of the periodic table is the element resulting from the emission of the third alpha particle?
A) 4A (14)
B) 5A (15)
C) 6A (16)
D) 7A (17)
E) 8A (18)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 9.52 × 10-5 mol sample of rubidium-86 emits 8.87 × 1016 β particles in one hour. What is the half-life of rubidium-86?
A) 2.23 × 10 -3 h
B) 1.55 × 10 -3 h
C) 448 h
D) 645 h
E) None of these choices are correct.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 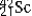 is unstable. This is predictable because
is unstable. This is predictable because
A) the number of neutrons is too large in relation to the number of protons.
B) the number of neutrons is too small in relation to the number of protons.
C) the atomic number is too large.
D) the mass number is too large.
E) Sc isotopes are all unstable.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following equations correctly represents positron decay of 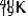 ?
?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 82
Related Exams