A) a compound with the same number of carbon atoms as the hormone
B) a compound with the same molecular mass (measured in daltons) as the hormone
C) a compound with the same three-dimensional shape as part of the hormone
D) a compound with the same number of orbital electrons as the hormone
E) a compound with the same number of hydrogen and nitrogen atoms as the hormone
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
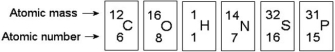 -How many neutrons are present in the nucleus of a phosphorus-32 (³²P) atom (see the figure above) ?
-How many neutrons are present in the nucleus of a phosphorus-32 (³²P) atom (see the figure above) ?
A) 5
B) 15
C) 16
D) 17
E) 32
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating how chemical reactions occur.You place two reactants together and measure the concentration of product at regular intervals.After a time,the amount of product becomes stable. -This solution has
A) used up all the reactants, so no more product can be made.
B) used up all the product, so no more reaction is occurring.
C) reached equilibrium, where there is no more formation of the product.
D) reached equilibrium, where the net formation of both product and reactants is neutral.
E) become saturated.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reactivity of an atom arises from
A) the average distance of the outermost electron shell from the nucleus.
B) the existence of unpaired electrons in the valence shell.
C) the sum of the potential energies of all the electron shells.
D) the potential energy of the valence shell.
E) the energy difference between the s and p orbitals.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms whose outer electron shells contain 8 electrons tend to
A) form ions in aqueous solutions.
B) form hydrogen bonds in aqueous solutions.
C) be stable and chemically nonreactive, or inert.
D) be gaseous at room temperature.
E) be both chemically inert and gaseous at room temperature.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly describes water's unique properties?
A) Hydrogen bonds in liquid water form a crystalline structure.
B) Water has a low heat of vaporization resulting in the evaporative cooling effect we experience when we sweat.
C) Water has a low specific heat resulting in a significant amount of heat being released when hydrogen bonds form.
D) Water is a non-polar molecule because oxygen and hydrogen have the same electronegativities.
E) Water molecules like to stick together due to hydrogen bonding.
G) B) and C)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
 -Which one of the atoms shown would be most likely to form a cation with a charge of +1?
-Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Chemical bond/interaction strength appears in what order?
A) Hydrogen > covalent > ionic > VanderWaals.
B) Covalent > ionic > hydrogen > VanderWaals.
C) Covalent > hydrogen > ionic > VanderWaals.
D) VanderWaals > hydrogen > ionic > covalent.
E) Ionic > hydrogen > VanderWaals > covalent.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of chlorine is 17.The atomic number of magnesium is 12.What is the formula for magnesium chloride?
A) MgCl
B) MgCl₂
C) Mg₂Cl
D) Mg₂Cl₂
E) MgCl₃
G) B) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Magnesium has an atomic number of 12.What is the most stable charge for a magnesium ion?
A) a +1 charge
B) a +2 charge
C) a -1 charge
D) a -2 charge
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not considered to be a weak molecular interaction?
A) a covalent bond
B) a van der Waals interaction
C) an ionic bond in the presence of water
D) a hydrogen bond
E) both a hydrogen bond and a covalent bond
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combining of the metal,sodium,with the poisonous gas,chlorine,to produce an edible product,salt,is a good example of
A) essential elements.
B) emergent properties.
C) covalent interactions.
D) Van der Waals interactions.
E) chemical equilibrium.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Based on electron configuration,which of these elements in the figure above would exhibit a chemical behaviour most like that of oxygen?
-Based on electron configuration,which of these elements in the figure above would exhibit a chemical behaviour most like that of oxygen?
A) carbon
B) hydrogen
C) nitrogen
D) sulphur
E) phosphorus
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphorus-32,a radioactive isotope of phosphorus-31 (atomic number 15) ,undergoes a form of radioactive decay whereby a neutron turns into a proton and emits radiation in the form of an electron.What is the product of such radioactive decay of phosphorus-32?
A) phosphorus-31
B) a positively charged phosphorus-31 ion
C) a negatively charged phosphorus-32 ion
D) sulphur-32 (atomic number 16)
E) the conversion of the phosphorus-32 atom into pure energy
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen has an atomic number of 8 and a mass number of 16.Thus,what is the atomic mass of an oxygen atom?
A) exactly 8 grams
B) exactly 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
E) 24 amu (atomic mass units)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does radiometric dating allow researchers to determine the age of fossils?
A) Radioactive isotopes are incorporated into living organisms easier than the corresponding non-radioactive isotope.
B) The half-life for all isotopes are all long, in the order of years.
C) The "parent" isotope decays into the "daughter" isotope at a fixed rate.
D) All radioactive isotopes have the same half-life.
E) All elements incorporated into living organisms have radioactive isotopes.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nucleus of a nitrogen atom contains 7 neutrons and 7 protons.Which of the following is a correct statement concerning nitrogen?
A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14.
B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7.
C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams.
D) The nitrogen atom has a mass number of 7 and an atomic number of 14.
E) The nitrogen atom has a mass number of 14 and an atomic mass of approximately 14 daltons.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
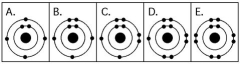 -Which drawing in the figure above depicts an atom with a valence of 3?
-Which drawing in the figure above depicts an atom with a valence of 3?
A) A
B) B
C) C
D) D
E) E
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly describes electrons?
A) An electron can move from one shell to another only if the energy the electron gains is greater than the difference in energy between the energy levels of the two shells.
B) Electrons are involved in the chemical reactions between atoms.
C) Electrons are neutral.
D) Electrons can move from the nucleus to higher energy levels when they absorb energy.
E) Protons, neutrons and electrons have equal mass.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of hydrogen atoms that can be covalently bonded in a molecule containing two carbon atoms?
A) 2
B) 3
C) 4
D) 6
E) 8
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 98
Related Exams