B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-carbon and bonding molecular orbitals of ethene, H2C=CH2?
A) C2sp3 + C2sp3, and C2p + C2p
B) C2sp2 + C2sp2, and C2sp2 + C2sp2
C) C2sp2 + C2sp2, and C2p + C2p
D) C2sp3 + C2sp3, and C2sp2 + C2sp2
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is trigonal planar?
A) boron trifluoride, BF3
B) methyl anion, CH3-
C) methane, CH4
D) ammonia, NH3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The percent s character in an sp2 hybridized orbital is approximately 33%.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a carboxylic ester?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2COOCH3
D) CH3CH2COCH3
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate H-C-O bond angle in formaldehyde, H2C=O?
A) 90
B) 109
C) 120
D) 180
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The formal charges in the complex should below are 0 on each H, -1 on N, and +1 on B. 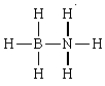
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an ionic bond?
A) F-F
B) C-H
C) Li-O
D) C-N
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Circle all of the sp2 hybridized atoms in the following molecular structure. 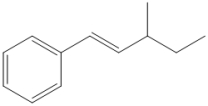
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the ketones that have the formula C5H10O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a tertiary alcohol?
A) CH3CH2OCH3
B) (CH3) 3COH
C) (CH3) 2CHOH
What is the correct assignment of the na
E) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The approximate H-C-H bond angle in methane is ______°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate C-C-C bond angle in propene, CH3CH=CH2?
A) 90
B) 109
C) 120
D) 180
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Different compounds with the same molecular formula are called __________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-hydrogen bonding molecular orbitals of ethene, H2C=CH2?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are there in the valence shell of the oxygen atom of water?
A) 2
B) 4
C) 6
D) 8
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Circle and name the functional groups in the following molecule. 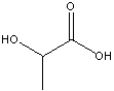
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the length of the C C bond in ethyne, HC CH?
A) 121 pm
B) 134 pm
C) 142 pm
D) 154 pm
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole moment?
A) CO2
B) BF3
C) NH3
D) CH4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
There are eight valence in a methyl anion, CH3-.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 115
Related Exams