A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams represents the slowest reaction? 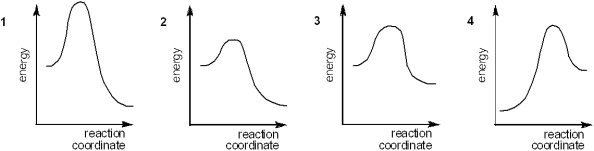
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
A reaction in which the free energy of the products is greater than the reactants is termed____________________ .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct order of decreasing basicity (stronger base > weaker base) ?
A) NH3 > MeNH2 > H2O > HF
B) MeNH2 > NH3 > MeOH > CH4
C) NH3 > Me3N > H2O > MeOH
D) CH3COONa > NaOH > NaOMe > NaNMe2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The structure of the alkaloid ephedrine is shown below. When ephedrine is placed in the presence of an acid, the -OH group will be protonated. 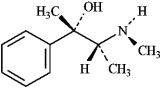
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pKa of acetic acid, CH3COOH, is 4.76. What is the value of the equilibrium constant Keq, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] ![The pK<sub>a</sub> of acetic acid, CH<sub>3</sub>COOH, is 4.76. What is the value of the equilibrium constant K<sub>eq</sub>, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] A) 3.2 * 10<sup>7</sup> B) -3.2 * 10<sup>-</sup><sup>7</sup> C) -3.2 * 10<sup>7</sup> D) 3.2 *10<sup>-</sup><sup>7</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1813/11ea7d75_f5e3_6f2f_b9bd_77533aa092af_TB1813_00_TB1813_00.jpg)
A) "3.2 * 107"
B) "-3.2 * 10-7"
C) "-3.2 * 107"
D) "3.2 *10-7"
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has a pKa value of approximately 25?
A) CH3CH3
B) CH2=CH2
C) HC CH
D) CH3CH2OH
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is present in the highest concentration upon dissolution of acetic acid in water?
A) OH-
B) H3O+
C) CH3COOH
D) CH3COOH+
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a Lewis acid but not a Brønsted-Lowry acid?
A) CH3COOH
B) AlCl3
C) H2O
D) CH3OH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium? 
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following compounds is the strongest acid?
A) HF
B) H2O
C) NH3
D) CH4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following anions is the strongest base?
A) NH2-
B) NH3
C) CH3CH=N-
D) CH3C N
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the reactivity of trimethylamine, (CH3) 3N?
A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Lewis acid and not a Brønsted-Lowry acid
D) Lewis base and not a Brønsted-Lowry base
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following is a definition of the rate-determining step of a reaction mechanism?
A) the first step
B) the last step
C) the step that crosses the highest energy barrier
D) the most exothermic step
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation that relates the acid dissociation constant, Ka, to the pKa of an acid.
Correct Answer

verified
Correct Answer
verified
True/False
Consider the following reaction coordinate diagram. 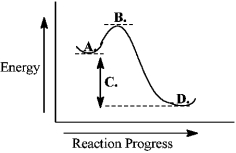 The free energy of activation is represented by the letter C.
The free energy of activation is represented by the letter C.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH3COOH
B) ClCH2COOH
C) CH3CH2OH
D) ClCH2CH2OH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a feature of a Lewis acid?
A) proton donor
B) proton acceptor
C) electron pair donor
D) electron pair acceptor
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following anions is the strongest base?
A) CH3COO-
B) HO-
C) NH2-
D) Cl-
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Which acid has the smallest Ka?
Correct Answer

verified
ethanol
Consider the following terms. Use these as appropriate to complete the following statements.
transition state
endergonic
exergonic
free energy of activation
Gibbs free energy changes
reaction intermediate
Correct Answer
verified
Showing 1 - 20 of 94
Related Exams