A) 2
B) 4
C) 6
D) 8
E) 10
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron in an atom has the following set of quantum numbers:
n = 2, 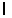 = 1,
= 1, 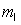 = -1, ms = +1/2.
-In which subshell can the electron be found?
= -1, ms = +1/2.
-In which subshell can the electron be found?
A) s
B) p
C) d
D) f
E) g
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an X-ray tube, electrons with energy 38 keV are incident on a cobalt (Z = 27) target. Determine the cutoff wavelength for X-ray production.
A) 1.9 × 10-11 m
B) 2.4 × 10-11 m
C) 2.8 × 10-11 m
D) 3.3 × 10-11 m
E) 3.6 × 10-11 m
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the following sentence: In the condition known as population inversion,
A) the amount of one type of gas atoms is larger than that of another in a mixture.
B) the number of energy levels that are populated is larger than that of unpopulated levels.
C) there are more electrons occupying lower energy levels than occupying higher energy levels.
D) there are more electrons occupying higher energy levels than occupying lower energy levels.
E) there are more photons than electrons in a given system.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the longest wavelength in the Paschen series of atomic spectra?
A) 8.204 × 10-7 m
B) 1.875 × 10-6 m
C) 2.216 × 10-6 m
D) 5.522 × 10-6 m
E) 6.756 × 10-5 m
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A pulsed laser has an average output power of 4.0 W. Each pulse consists of light at wavelength 5.0 × 10-7 m and has a 25 ms duration. How many photons are emitted in a single pulse?
A) 1.0 × 1017
B) 2.5 × 1017
C) 3.7 × 1017
D) 5.0 × 1017
E) 7.4 × 1017
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters. 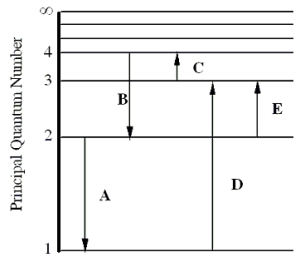 Note: The diagram is not drawn to scale.
-Determine the wavelength of the radiation involved in transition B.
Note: The diagram is not drawn to scale.
-Determine the wavelength of the radiation involved in transition B.
A) 291 nm
B) 364 nm
C) 487 nm
D) 652 nm
E) 1910 nm
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the following statement: An individual copper atom emits electromagnetic radiation with wavelengths that are
A) evenly spaced across the spectrum.
B) unique to that particular copper atom.
C) the same as other elements in the same column of the periodic table.
D) unique to all copper atoms.
E) the same as those of all elements.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following electronic configurations corresponds to an atomic ground state?
A) 1s2 2s1 2p6
B) 1s1 2s1 2p1
C) 1s1 2s2 3p1
D) 1s2 2s2 2p1
E) 1s1 2s2 2p1
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the maximum wavelength of incident radiation that can be used to remove the remaining electron from a singly ionized helium atom He+ (Z = 2) . Assume the electron is in its ground state.
A) 6.2 nm
B) 12.4 nm
C) 22.8 nm
D) 45.6 nm
E) 54.4 nm
G) A) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Complete the following statement: An h subshell refers to orbital quantum number
A) ![]() = 1.
= 1.
B) ![]() = 2.
= 2.
C) ![]() = 3.
= 3.
D) ![]() = 4.
= 4.
E) ![]() = 5.
= 5.
G) A) and C)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which model of atomic structure was developed to explain the results of the experiment shown? 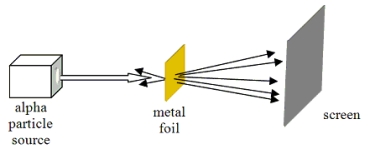
A) Bohr model
B) quantum mechanical atom
C) billiard ball atom
D) plum-pudding model
E) nuclear atom
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom will emit photons when one of its electrons goes from
A) the K shell to the L shell.
B) the M shell to the N shell.
C) the K shell to the M shell.
D) the N shell to the L shell.
E) the K shell to the N shell.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters.  Note: The diagram is not drawn to scale.
-In which transition is a Balmer series photon absorbed?
Note: The diagram is not drawn to scale.
-In which transition is a Balmer series photon absorbed?
A) A
B) B
C) C
D) D
E) E
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ground state electronic configuration of a neon atom is 1s2 2s2 2p6. How many of these electrons have magnetic quantum number ml = 0?
A) 2
B) 4
C) 6
D) 8
E) 10
G) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Determine the kinetic energy of an electron that has a de Broglie wavelength equal to twice the diameter of the hydrogen atom. Assume that the hydrogen atom is a sphere of radius 5.3 × 10-11 m.
A) 13.6 eV
B) 27.2 eV
C) 33.6 eV
D) 48.9 eV
E) 65.2 eV
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the Bohr model to estimate the K X-ray wavelength for a gold atom (Z = 79) .
A) 5.13 × 10-10 m
B) 8.54 × 10-10 m
C) 2.00 × 10-11 m
D) 3.60 × 10-11 m
E) 2.47 × 10-13 m
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why was it necessary for Bohr to require that electrons remain in stationary orbits?
A) An electron must travel in a circular path.
B) It was required by the Heisenberg uncertainty principle.
C) No two electrons can be in the same region in the atom.
D) It was required by the Pauli exclusion principle.
E) Classical physics predicts that the electron should spiral into the nucleus.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the shortest possible wavelength in the Lyman series for atomic hydrogen?
A) 91.3 nm
B) 104 nm
C) 122 nm
D) 364 nm
E) 820 nm
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the planetary model of the atom where electrons orbit a centralized nucleus, what is the approximate ratio of the radius of the nucleus to that of the electron orbits, rn/re?
A) 105
B) 10-3
C) 103
D) 10-5
E) 10-7
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 74
Related Exams